
The temperature is obviously not a measure of disorder, since fusion and boiling, representing large increases in disorder, take place at constant temperature. A still larger increase in disorder occurs at the boiling point. At the melting point the substance absorbs heat at constant temperature forming a liquid far less ordered than the solid. Because of the vibrations atomic and molecular distances will fluctuate, and the crystal is more disordered. Atoms and molecules will increase their vibrations as temperature increases. Heat absorbed by the crystal leads to disorder, e.g., in form of vibrations. The third law of thermodynamics states that the entropy Is equal to zero for a perfect crystal at zero Kelvin: Since there is no disorder, the entropy is assumed to be zero. A perfect crystal at zero temperature kelvin is completely ordered. We can also interpret other entropy changes in terms of disorder in molecular dimensions. Whenever there are forces between molecules or particles, the condition of maximum probability is a balance between the two tendencies above. In the ideal gas there are no forces between the molecules, therefore the condition of maximum probability is complete disorder. (4.8), ∆ fusS = (1/T∆ fusH, expresses the balance between the tendency towards disorder and the tendency towards a low potential energy.
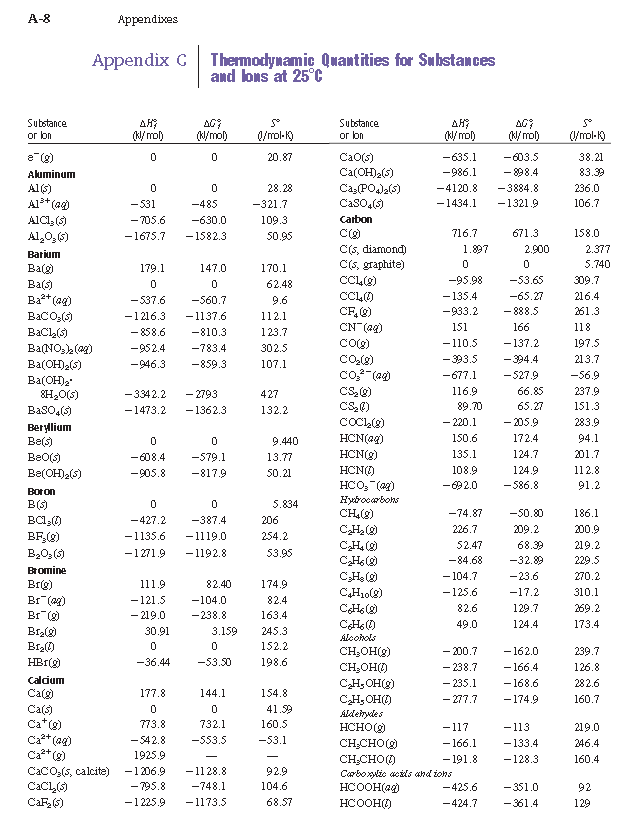
Entropy table free#
The water molecules are to some degree free to move about at random. In solid ice the water molecules are in fixed positions of an ordered lattice, while in liquid water there is much less order. An increase in entropy was found, and this means that the results of melting ice is more disorder. The entropy change upon melting of ice was studied in lesson 4, example 1. e.g When we boil a litre of water we can obtain five litres of steam which is obviously a more disordered state That is, when thermal energy is added to a system, it becomes more random, more disordered. The probability is related to order - disorder, and the entropy of a system is a measure of its randomness. The second law of thermodynamics can be expressed in terms of probability. LESSON DEVELOPMENT Probability and disorder Appreciate why one can calculate absolute entropies but not absolute values of other state functions like enthalpy, H.Calculate entropy changes for chemical reactions.Access standard entropies from physical standard tables.TITLE : ABSOLUTE VALUES OF ENTROPIES LEARNING OUTCOMES:Īt the end of the Lesson the students should be able to:



 0 kommentar(er)
0 kommentar(er)
